Many references are available, including the book:
"Microwave Techniques and Protocols", Demaree and Giberson, 2001 (
Prod. No. 24940)
Research by Sanders and Gartner resulted in a very interesting paper in the above
book describing successful in vivo nuclear labeling of both Allium sp. root tip and
Drosophila melanogaster embryos using low power (~250W) and the
PELCO ColdSpot®. This paper is evidence to support microwave
activity in contrast to sole dependency on heat effects.
|
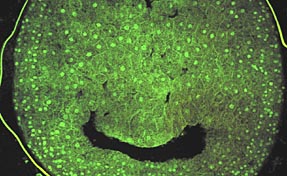 |
Microwave-assisted labeling using Sytox (Molecular Probes, Inc.) nucleic acid stain
on vibratome section of chicken embryo. Fixed vibratome sections were incubated in
2 mM Sytox for 2 minutes at 200 Watts, 2 minutes without microwave irradiation, and
2 minutes at 200 Watts microwave incubation under continuous 15in. Hg vacuum. Optical
sections were collected on a BioRad 1024 laser scanning confocal microscope using
488nm excitation wavelength. Digital projections were made from the ~50mm thick optical
sections to create the plate.
Mark A. Sanders, Imaging Center, University of Minnesota.
|
Diagnostic EM
Giberson RT, Austin RL, Charlesworth J, Adamson G, Herrera GH, 2002. Microwave and
Digital Imaging Technology: Reduce Turnaround Times for Diagnostic Electron Microscopy.
Ultrastructure Pathology (in press).
Giberson RT, Demaree Jr RS, Nordhausen RW, 1997. Four-hour processing of clinical/diagnostic specimens for electron microscopy. J Vet Diagn Invest 9:61-67.
|
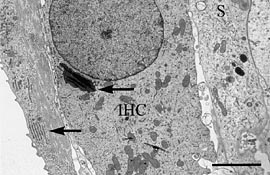 |
An inner hair cell (IHC) with supporting cells (S) from a Japanese macaque monkey
cochlea decalcified using microwave methods. The arrows indicate rough endoplasmic
reticulum and golgi apparatus. Bar = 3.0µm.
Reprinted from Madden VJ and Henson NM, Hearing Research, Volume 111, issues 1-2, 1997.
|
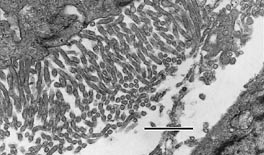 |
The characteristic long, narrow, undulating microvilli are easily identified from
this mesothelioma of the pleura processed by microwave methods for diagnostic electron
microscopy, described by Munn RJ and Vogt PJ, In: Microwave Techniques and Protocols,
2001 (
Prod. No. 24940). Robert Munn, School of Medicine, UC Davis, Davis, CA. Bar = 1.0µm
|
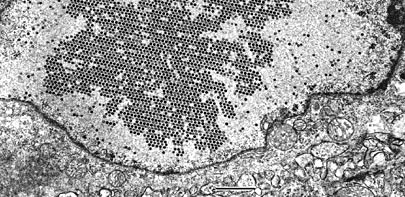 |
A turkey tubular epithelial cell with adenovirus nephritis. The tissue was processed
by microwave methods for electron microscopy, described by Nordhausen RW and Barr
BC, In: Microwave Techiques and Protocols, 2001 (
Prod. No. 24940). The nucleus of the infected cell shows an intranuclear paracrystalline adenovirus inclusion. Bar = 1.0µm. Bob Nordhausen, California Animal Health and Food Safety Laboratory, UC Davis, Davis, CA.
|
Electron Microscopy
Block-Alper L, Webster P, Zhou X, Supekova L, Wong WH, Schultz PG, Meyer DI, 2002. IN02, a positive regulator of lipid biosynthesis, is essential for the formation of inducible membranes in yeast. Mol Cell Bio 13(1):40-51.
von Dohlen CD, Kohler S, Alsop ST, McManus WR, 2001. Mealybug ß-proteobacterial
endosymbionts contain g- proteobacterial symbionts. Nature 412:433-436.
Giberson RT, Demaree Jr RS, 1999. Microwave processing techniques for electron microscopy: A four-hour protocol. In: Electron Microscopy Methods and Protocols, Hajibagheri N, ed. Humana Press, Inc., Totowa, NJ, pp 145-158
.
Fiala JC, Feinberg M, Popov V, Harris KM, 1998. Synaptogenesis via dendritic filpodia in developing hippocampal area CA1. J Neurosci 18: 8900-8911.
Demaree, R.S., Jr., Giberson, R.T., Smith, R.L. (1995) Routine microwave polymerization
of resins for transmission electron microscopy. Scanning 17(Suppl. 5):25-26.
Giberson, R.T., Smith, R.L., Demaree, R.S. (1995) Three hour microwave tissue processing
for transmission electron microscopy: from unfixed tissues to sections. Scanning
17(suppl. 5):26-27.
Giberson, R.T., Demaree, R.S., Jr. (1995) Microwave fixation: understanding the variables to achieve rapid reproducible results. Micros Res Tech 32:246-254.
Histology
Rassner UA, Crumrine DA, Nau P, Elias PM, 1997. Microwave incubation improves lipolytic enzyme preservation for ultrastructural cytochemistry. Histochem J 29:387-392.
Schray CL, Metz AL, Gough AW, 2002. Microwave-Enhanced Fixation for Rapid Preparation of Tissue Sections for Microscopic Evaluation. Histologic 35:1, pp. 7-12.
|
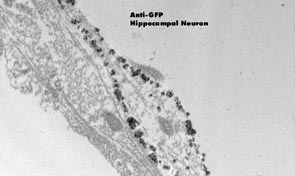 |
Microwave processed and immunolabeled hippocampal neurons in culture. Hippocampal
neuron labeled with anti-GFP primary and silver enchanced gold secondary demonstrates
the membrane distribution of the protein. Buchanan et al., Microwave Processing and
Pre-embedding Nanogold Immunolabeling for Electron Microscopy, Micros Microanalysis,
8(Suppl. 2):160-1, 2002.
|
Immunolabeling
Chicoine L, Webster P, 1998. The effect of microwave irradiation on antibody labeling efficiency when applied to ultrathin cryosections through fixed biological material. Micros Res Tech 42, pp. 24-32.
|
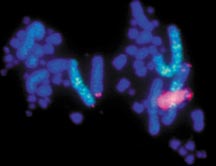 microwave-assisted in situ hybridization on pig chromosome preparation. labeling done with a pelco® microwave processor utilizing the
PELCO ColdSpot® to regulate temperature and microwave energy distribution. microwave-assisted in situ hybridization on pig chromosome preparation. labeling done with a pelco® microwave processor utilizing the
PELCO ColdSpot® to regulate temperature and microwave energy distribution.
Processing completed in ~4 hours.
|
Immunostaining
Micheva KD, Holz RW, Smith SJ, 2001. Regulation of presynaptic phosphatidy linositol 4,5-biphosphate by neuronal activity. J Cell Bio 154: 355-368.
Petrali JP, Mills KR, 1998. Microwave-assisted immunoelectron microscopy of skin.
Micros Microanalysis 4 (suppl 2:Proceedings), pp. 114-115.
Immunocytochemistry
Madden VJ, 1998. Microwave processing of cell monolayers in situ for post-embedding
immunocytochemistry with retention of ultrastructure and antigenicity. Micros Microanalysis
4 (Suppl 2:Proceedings): 854-55.
Rangell LK, Keller GA, 2000. Application of microwave technology to the processing and imunolabeling of plastic-embedded cytosections. J Histochem Cytochem 48:1153-1160.
Schichnes D, Nemson J, Sohlberg L, Ruzin SE, 1999. Microwave protocols for paraffin Microtechnique and in situ localization in plants. Micros Microanalysis 4:491-496.
|