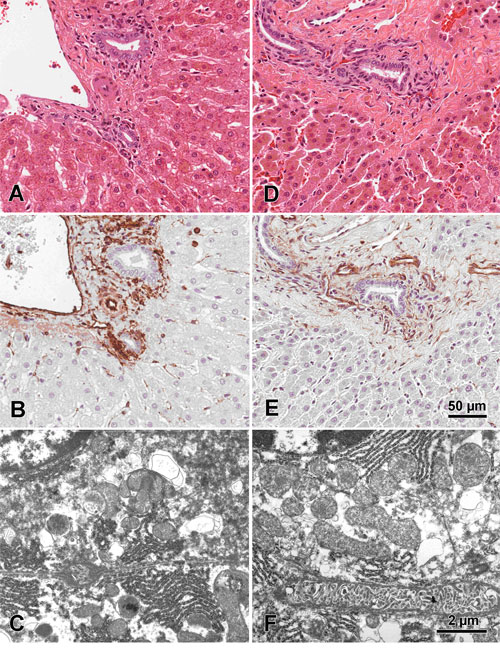
|
Figure 4A-F. Figures A-C are of normal mouse liver benched fixed in 10% NBF for 24 hours. Figures D-F
are of normal mouse liver fixed in 10% NBF for 20 minutes utilizing microwave radiation.
All tissues were prepared for
fixation identically and cut to 2mm prior to fixation. Figures A and D are corresponding
Hematoxylin and Eosin stained
sections and figures B and E are corresponding Vimentin IHC stained sections. Figures
C and F are corresponding EM sections
demonstrating complimentary ultra-structure. Images are from Dr. Jose Galvez, Center
for Comparative Medicine and Department
of Medical Pathology, University of California, Davis, CA.
|
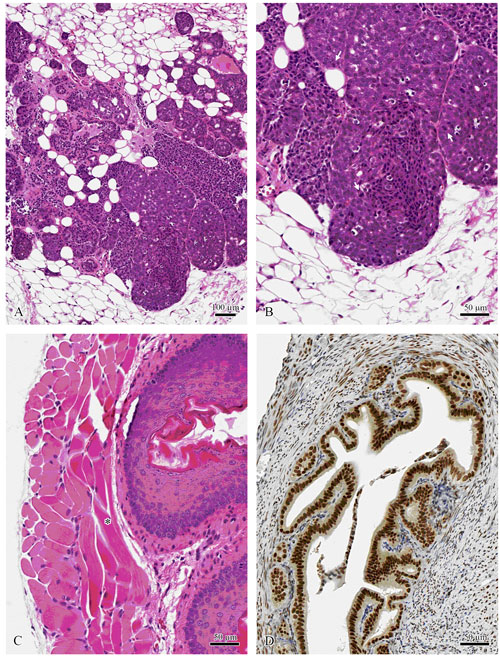
|
Figure 5. Tissues formalin fixed and paraffin processed by the protocols described in Galvez et al. 2006.
Mouse mammary tumor virus induced mammary carcinoma (A, B). Note the mitotic figures
(arrows) in B. Mouse esophagus with clearly identifiable muscle striations (*) (C).
Mouse uterus stained with mouse anti-estrogen (D). (Center for Comparative
Medicine and Department of Pathology, Univ. of Calif., Davis)
|
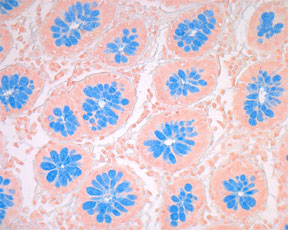
Special Stains
Mucin – Alcian Blue pH 2.5 - Microwave Procedure1
Notes:
- Place temperature probe in the PELCO ColdSpot® port when Temperature Restriction
(TR) is "None" and set TR to >50ºC
- Place sample container on the PELCO ColdSpot®
- Check wattage setting
- LED above wattage setting indicator will be RED when the magnetron is on
- When a TR is set the LED will be RED until the TR is reached then it will turn GREEN (magnetron off) and cycle between RED and GREEN for the remaining time
- Place temperature probe in sample container and set TR
|
 |
| Step |
Container Reagent |
Wattage Setting |
Temperature Restriction |
Time/Pad# |
| 1 |
50ml Coplin Jar
xylene
Note #2 |
165W - #1
Note #3 |
None
Note #1 |
4 min. - #8
Note #4 |
| 2 |
50ml Coplin Jar
95-100% ETOH
Note #2 |
165W - #1
Note #3 |
None
Note #1 |
1 min. - #3
Note #4 |
| 3 |
Wash in tap water to clear |
BENCH STEP |
|
|
| 4 |
50ml Coplin Jar
3% Acetic Acid
Notes #2 & #6 |
165W - #1
Note #3 |
TR 45°C
Note #5 |
30 sec. - #1
Note #4 |
| 5 |
50ml Coplin Jar
Alcian Blue Sol.
Notes #2 & #6 |
165W - #1
Note #3 |
TR 45°C
Note #5 |
1 min. 30 sec.
Note #4 |
| 6 |
Wash in DI water 3 changes |
BENCH STEP |
|
|
| 7 |
50ml Coplin Jar
Nuclear Fast Red*
Notes #2 & #6 |
165W - #1
Note #3 |
TR 45°C
Note #5 |
1 min. 30 sec.
Note #4 |
| 8 |
Wash in DI water 3 changes |
BENCH STEP |
|
|
| 9 |
Dehydrate through ETOH's,
clear and mount |
BENCH STEP |
|
|
| NOTES Steps #4, #5 and #7 do not require reaching 45ºC |
*Or Brazilliant, Anatech
1Requires a microwave with continuous variable wattage and a PELCO ColdSpot® - Recommended models are a PELCO BioWave®
REFERENCES:
Carson,F.L., Histotechnology: A Self-Instructional Text, ASCP Press, 1990, pg 130 - 131
Sheehan and Hrapchak, Theory and Practice of Histotechnology, 2nd ed.1980, Mosby
Preece, A Manual for Histologic Technicians, 3rd ed.,1972, Little, Brown & Co.
RESULTS:
Exclusively acid mucosubstances - blue
Nuclei - pale red.
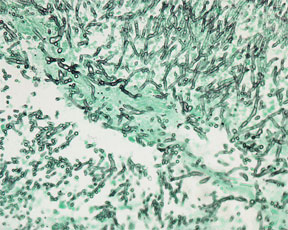
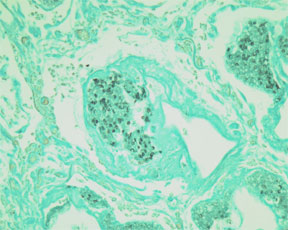
Fungus & Pneumocystis – Grocott's Methenamine Silver Nitrate - Microwave Procedure1
Notes:
- Place temperature probe in the PELCO ColdSpot® port when Temperature Restriction (TR) is "None" and set TR to >50ºC
- Place sample container on the PELCO ColdSpot®
- Check wattage setting
- LED above wattage setting indicator will be RED when the magnetron is on
- When a TR is set the LED will be RED until the TR is reached then it will turn GREEN (magnetron off) and cycle between RED and GREEN for the remaining time
- Place temperature probe in sample container and set TR
|
 |
 |
| Step |
Container Reagent |
Wattage Setting |
Temperature Restriction |
Time/Pad# |
| 1 |
50ml Coplin Jar
xylene
Note #2 |
165W - #1
Note #3 |
None
Note #1 |
4 min. - #8
Note #4 |
| 2 |
50ml Coplin Jar
95-100% ETOH
Note #2 |
165W - #1
Note #3 |
None
Note #1 |
1 min. - #3
Note #4 |
| 3 |
Wash in tap water to clear |
BENCH STEP |
|
|
| 4 |
50ml Coplin Jar
5% Chromic Acid
Notes #2 & #6 |
502W - #3
Note #3 |
TR 60°C
Note #5 |
6 min. - #0
Note #4 |
| 5 |
Wash in tap water |
BENCH STEP |
|
|
| 6 |
50ml Coplin Jar
sodium bisulfite sol.
|
BENCH STEP |
|
1 min. |
| 7 |
Wash in tap
then 3-4 times in DI water |
BENCH STEP |
|
|
| 8 |
50ml Coplin Jar
Methenamine silver
Notes #2 & #6
|
315W - #2
Note #3 |
TR 60°C
Note #5 |
4 min. - 30 sec. - #9
Note #4 |
| 9 |
Wash in tap water
then 3-4 changes DI |
BENCH STEP |
|
|
| 10 |
50ml Coplin Jar
1% gold chloride
Notes #2 & #6 |
165W - #1
Note #3 |
TR 40ºC
Note #5 |
40 sec. - #2
Note #4 |
| 11 |
Rinse in DI water |
BENCH STEP |
|
|
| 12 |
50ml Coplin Jar
2% sodium thiosulfate
Notes #2 & #6 |
165W - #1
Note #3 |
TR 40ºC
Note #5 |
40 sec. - #2
Note #4 |
| 13 |
Rinse in Tap
then
DI water |
BENCH STEP |
|
|
| 14 |
50ml Coplin Jar
Light green solution
Notes #2 & #6 |
165W - #1
Note #3 |
TR 40ºC
Note #5 |
40 sec. - #2
Note #4 |
| 15 |
Dehydrate through ETOH's,
clear and mount |
BENCH STEP |
|
|
NOTES
Step #8 should reach 60ºC in the first 2 min. of the time period.
1. Adjust wattage to smooth heating curve.
2. Repeat step for 2 min. or less to improve silver development. Keep the TR at 60ºC. |
NOTES
Steps #10, 12 and 14 do not need to reach 40ºC in the time period. |
*Or Brazilliant, Anatech
1Requires a microwave with continuous variable wattage and a PELCO ColdSpot® - Recommended models are a PELCO BioWave®
REFERENCES:
Sheehan and Hrapchak, Theory and Practice of Histotechnology, 2nd ed.1980, Mosby pg. 245
Preece, A Manual for Histologic Technicians, 3rd ed., 1972, Little, Brown & Co. page 335, with modifications.
RESULTS:
Fungi and Pneumocystis - dark brown to black
Mucin - taupe to dark gray
Background - light green
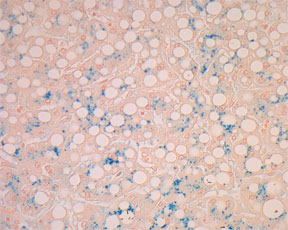
Iron-Prussian Blue Stain for Ferric Iron - Microwave Procedure1
Notes:
- Place temperature probe in the PELCO ColdSpot® port when Temperature Restriction
(TR) is "None" and set TR to >50ºC
- Place sample container on the PELCO ColdSpot®
- Check wattage setting
- LED above wattage setting indicator will be RED when the magnetron is on
- When a TR is set the LED will be RED until the TR is reached then it will turn GREEN (magnetron off) and cycle between RED and GREEN for the remaining time
- Place temperature probe in sample container and set TR
|
 |
| Step |
Container Reagent |
Wattage Setting |
Temperature Restriction |
Time/Pad# |
| 1 |
50ml Coplin Jar
xylene
Note #2 |
165W - #1
Note #3 |
None
Note #1 |
4 min. - #8
Note #4 |
| 2 |
50ml Coplin Jar
95-100% ETOH
Note #2 |
165W - #1
Note #3 |
None
Note #1 |
1 min. - #3
Note #4 |
| 3 |
Wash in tap water to clear |
BENCH STEP |
|
|
| 4 |
Wash in DI water 3 changes |
BENCH STEP |
|
|
| 5 |
50ml Coplin Jar
Ferrocyanide/HCl
Notes #2 & #6 |
315W - #2
Note #3 |
TR 45°C
Note #5 |
2 min. - #6
Note #4 |
| 6 |
Wash in DI water 3 changes |
BENCH STEP |
|
|
| 7 |
50ml Coplin Jar
Nuclear Fast Red*
Notes #2 & #6 |
315W - #2
Note #3 |
TR 45°C
Note #5 |
1 min. 30 sec. - #5
Note #4 |
| 8 |
Wash in DI water 3 changes |
BENCH STEP |
|
|
| 9 |
Dehydrate through ETOH's,
clear and mount |
BENCH STEP |
|
|
NOTES
Step #5 and #7 do not require reaching 45ºC |
*Or Brazilliant, Anatech
1Requires a microwave with continuous variable wattage and a PELCO ColdSpot® - Recommended models are a PELCO BioWave®
REFERENCES:
Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology, 3rd ed., Lee Luna (editor), 1968
Carson, F.L., Histotechnology: A Self-Instructional Text, ASCP Press, 1990
RESULTS:
Hemosiderin (iron) - blue
Nuclei and hemofuchsin - red
Background - pink
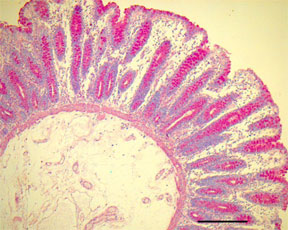
PAS – Periodic Acid-Schiff Reaction – Microwave Procedure1
Notes:
- Place temperature probe in the PELCO ColdSpot® port when Temperature Restriction
(TR) is "None" and set TR to >50ºC
- Place sample container on the PELCO ColdSpot®
- Check wattage setting
- LED above wattage setting indicator will be RED when the magnetron is on
- When a TR is set the LED will be RED until the TR is reached then it will turn GREEN (magnetron off) and cycle between RED and GREEN for the remaining time
- Place temperature probe in sample container and set TR
|
 |
| Step |
Container Reagent |
Wattage Setting |
Temperature Restriction |
Time/Pad# |
| 1 |
50ml Coplin Jar
xylene
Note #2 |
165W - #1
Note #3 |
None
Note #1 |
4 min. - #8
Note #4 |
| 2 |
50ml Coplin Jar
95-100% ETOH
Note #2 |
165W - #1
Note #3 |
None
Note #1 |
1 min. - #3
Note #4 |
| 3 |
Wash in tap water to clear |
BENCH STEP |
|
|
| 4 |
50ml Coplin Jar
0.5% Periodic Acid
Notes #2 & #6 |
315W - #2
Note #3 |
TR 60°C
Note #5 |
2 min. 30 sec. - #7
Note #4 |
| 5 |
Wash in DI water 3 changes |
BENCH STEP |
|
|
| 6 |
50ml Coplin Jar
Schiff Reagent*
Notes #2 & #6 |
165W - #1
Note #3 |
TR 45°C
Note #5 |
2 min. - #6
Note #4 |
| 7 |
50ml Coplin Jar
Warm Tap Water
Notes #2 & #6 |
165W - #1
Note #3 |
TR 45°C
Note #5 |
4 min. - #8
Note #4 |
| 8 |
Wash in tap water |
BENCH STEP |
|
|
| 9 |
50ml Coplin Jar
Gill #1 Hematoxylin
Notes #2 & #6 |
165W - #1
Note #3 |
TR 45°C
Note #5 |
40sec. - #2
Note #4 |
| 10 |
Wash in DI water |
BENCH STEP |
|
|
| 11 |
Blue in Scott's water |
BENCH STEP |
|
1 min. |
| 12 |
Wash in tap water |
BENCH STEP |
|
|
| 13 |
Dehydrate through ETOH's,
clear and mount |
|
|
|
NOTES
Step #4 should approach 60ºC
Steps #6 to #8 do not require reaching 45ºC |
*Use Fresh Reagent – Destain with 0.55% Potassium Metabisulfite if needed after step #6
1Requires a microwave with continuous variable wattage and a PELCO ColdSpot® - Recommended models are a PELCO BioWave®
REFERENCES:
Carson, F.L., Histotechnology: A Self-Instructional Text, ASCP Press, 1990, pg 119
Sheehan and Hrapchak, Theory and Practice of Histotechnology, 2nd ed.1980, Mosby pg.164
"A Simple Method to Restore Exhausted Schiff Reagent", The Journal of Histotechnology, Vol. 20, No. 1, March 1997, pg. 79
RESULTS:
Glycogen, neutral mucosubstances, certain epithelial sulfomucin and sialomucins, colloid material of the thyroid and pars intermedia of the pituitary, basement membranes, and fungal walls - magenta or rose to red
Nuclei - blue
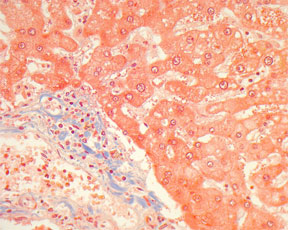
Collagen & Smooth Muscle – Masson's Trichrome Stain - Microwave Procedure1
Notes:
- Place temperature probe in the PELCO ColdSpot® port when Temperature Restriction
(TR) is "None" and set TR to >50ºC
- Place sample container on the PELCO ColdSpot®
- Check wattage setting
- LED above wattage setting indicator will be RED when the magnetron is on
- When a TR is set the LED will be RED until the TR is reached then it will turn GREEN (magnetron off) and cycle between RED and GREEN for the remaining time
- Place temperature probe in sample container and set TR
|
 |
| Step |
Container Reagent |
Wattage Setting |
Temperature Restriction |
Time/Pad# |
| 1 |
50ml Coplin Jar
xylene
Note #2 |
165W - #1
Note #3 |
None
Note #1 |
4 min. - #8
Note #4 |
| 2 |
50ml Coplin Jar
95-100% ETOH
Note #2 |
165W - #1
Note #3 |
None
Note #1 |
1 min. - #3
Note #4 |
| 3 |
Wash in tap water to clear |
BENCH STEP |
|
|
| 4 |
50ml Coplin Jar
Bouin's Solution
Notes #2 & #6 |
315W - #2
Note #3 |
TR 60°C
Note #5 |
6 min. - #0
Note #4 |
| 5 |
Wash in tap water to clear yellow |
BENCH STEP |
|
|
| 6 |
50ml Coplin Jar
Gill #2 Hematoxylin
Notes #2 & #6 |
315W - #2
Note #3 |
TR 40°C
Note #5 |
1 min. 20 sec. - #4
Note #4 |
| 7 |
50ml Coplin Jar
Wash in tap water to blue |
BENCH STEP |
|
|
| 8 |
50ml Coplin Jar
Biebrich Scarlet – Acid Fuchsin
Notes #2 & #6 |
315W - #2
Note #3 |
TR 40°C
Note #5 |
40sec. - #2
Note #4 |
| 9 |
Rinse in DI water
3 changes |
BENCH STEP |
|
|
| 10 |
50ml Coplin Jar
Phosphotungstic –
Phosphomolybdic Sol
Notes #2 & #6 |
315W - #2
Note #3 |
TR 40°C
Note #5 |
1 min. - #3
Note #4 |
| 11 |
50ml Coplin Jar
Analine Blue Solution
Notes #2 & #6 |
165W - #2
Note #3 |
TR 40°C
Note #5 |
40sec. - #2
Note #4 |
| 12 |
Rinse in tap water
then DI water
|
BENCH STEP |
|
|
| 13 |
50ml Coplin Jar
1% Acetic Acid Sol. |
BENCH STEP |
|
30 sec. |
| 14 |
Dehydrate through ETOH's,
clear and mount |
BENCH STEP |
|
|
NOTES
Step #4 should reach 60ºC in 3 to 4 min.
Step #6, 8, 10 and 11 do not require reaching 40ºC |
*Use Fresh Reagent – Destain with 0.55% Potassium Metabisulfite if needed after step #6
1Requires a microwave with continuous variable wattage and a PELCO ColdSpot® - Recommended models are a PELCO BioWave®
REFERENCES:
Sheehan and Hrapchak, Theory and Practice of Histotechnology, 2nd ed.1980, Mosby
F.L. Carson, Histotechnology: A Self-Instructional Text, ASCP Press, 1990
RESULTS:
nuclei - blue-black
collagen - blue
cytoplasm, keratin, muscle fibers, intercellular fibers - red